Khp naoh net ionic equation – The KHP-NaOH net ionic equation takes center stage in this captivating exploration, inviting readers to witness a meticulously crafted narrative that unfolds with grace and clarity. This equation, a cornerstone of analytical chemistry, orchestrates a fascinating dance between potassium hydrogen phthalate (KHP) and sodium hydroxide (NaOH), revealing the intricacies of acid-base reactions.
As we delve into the depths of this chemical encounter, we will dissect the balanced chemical equation, identify the elusive spectator ions, and unveil the net ionic equation that encapsulates the essence of this reaction. Along the way, we will uncover the significance of this reaction in the realm of acid-base titrations, shedding light on its indispensable role in determining the concentration of unknown acids.
KHP and NaOH Reaction Overview
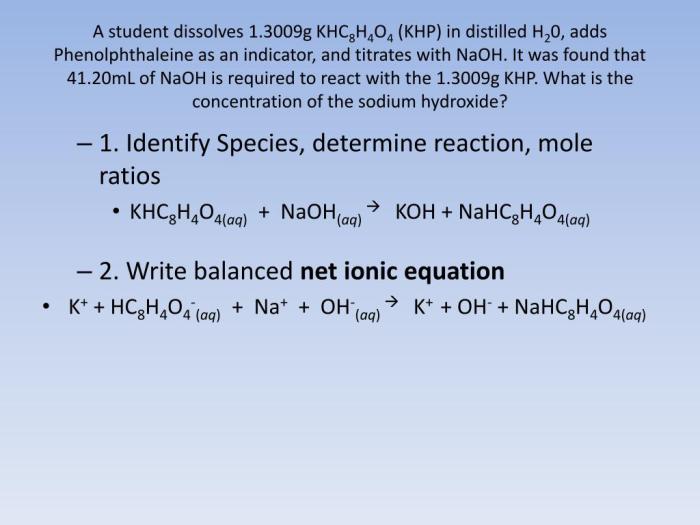
Potassium hydrogen phthalate (KHP) is a weak acid that reacts with sodium hydroxide (NaOH), a strong base, in a neutralization reaction. This reaction is commonly used in analytical chemistry for the standardization of NaOH solutions.
The balanced chemical equation for the reaction is:
KHC8H 4O 4(aq) + NaOH(aq) → NaKC 8H 4O 4(aq) + H 2O(l)
Net Ionic Equation
A net ionic equation shows only the ions that are directly involved in a chemical reaction. Spectator ions, which are ions that do not participate in the reaction, are excluded from the equation.
In the reaction between KHP and NaOH, the spectator ions are potassium (K+) and acetate (CH 3COO –). The net ionic equation is:
Reactants, Khp naoh net ionic equation
- K ++ H 2PO 4–
- Na ++ OH –
Products
- K ++ CH 3COO –
- Na ++ H 2PO 4–
Significance of the Reaction

The KHP-NaOH reaction holds great significance in analytical chemistry, primarily due to its crucial role in acid-base titrations. It serves as a reliable and accurate method for determining the concentration of unknown acids.
Acid-Base Titrations
In acid-base titrations, the KHP-NaOH reaction is employed to neutralize an unknown acid solution using a known concentration of NaOH solution. The equivalence point, where the moles of acid and base are equal, is determined through a color change of an indicator or by using instrumental methods.
This information allows for the calculation of the unknown acid’s concentration.The KHP-NaOH reaction is advantageous in acid-base titrations because KHP is a weak acid with a well-defined molar mass, making it easy to prepare solutions of precise concentrations. Additionally, the reaction proceeds quickly and stoichiometrically, ensuring accurate results.
The KHP-NaOH net ionic equation illustrates the reaction between potassium hydrogen phthalate (KHP) and sodium hydroxide (NaOH). This reaction is a classic example of acid-base neutralization. To enhance your understanding of chemical reactions, you may find the Crucible Study Guide Act 4 helpful.
It provides a comprehensive analysis of the play’s themes, characters, and historical context. Returning to the KHP-NaOH net ionic equation, we observe that the reaction produces potassium sodium tartrate (KNaC4H4O6) and water (H2O).
Experimental Considerations
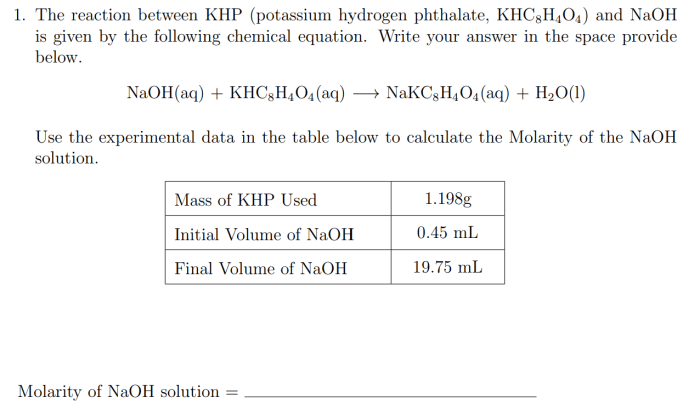
Performing the KHP-NaOH titration involves a specific experimental setup and the use of indicators to accurately determine the endpoint of the reaction.
The experimental setup typically includes a burette, a flask, a magnetic stirrer, and a pH meter or indicator solution. The burette is used to dispense the NaOH solution, while the flask contains the KHP solution. The magnetic stirrer ensures thorough mixing of the reactants, and the pH meter or indicator solution helps determine the endpoint of the titration.
Indicators in Titration
Indicators are substances that change color depending on the pH of the solution. In the KHP-NaOH titration, an indicator is added to the KHP solution before the titration begins. As the NaOH solution is added, the pH of the solution increases, and the indicator changes color when the equivalence point is reached.
This color change signals the endpoint of the titration, indicating that the moles of NaOH added are stoichiometrically equivalent to the moles of KHP present.
Calculations and Data Analysis

Once the titration is complete, you can calculate the concentration of the unknown acid using the following steps:
- Calculate the moles of NaOH used in the titration. To do this, multiply the volume of NaOH used (in liters) by its concentration (in moles per liter).
- Since the reaction between KHP and NaOH is 1:1, the moles of NaOH used are equal to the moles of KHP that reacted.
- Calculate the molarity of the unknown acid. To do this, divide the moles of KHP that reacted by the volume of the unknown acid used (in liters).
Sources of Error in the Titration
There are several sources of error that can affect the accuracy of the titration, including:
- Inaccurate measurement of the volume of NaOH used
- Inaccurate measurement of the mass of KHP used
- Incomplete reaction between KHP and NaOH
- Presence of impurities in the KHP or NaOH
To minimize the impact of these errors, it is important to use accurate equipment, carefully measure the reagents, and perform the titration slowly and carefully.
Applications in Real-World Scenarios: Khp Naoh Net Ionic Equation
The KHP-NaOH reaction finds diverse applications in various fields, offering numerous benefits and limitations.
Analytical Chemistry
-
-*Standardization of NaOH solutions
KHP serves as a primary standard for accurately determining the concentration of NaOH solutions. This is crucial in analytical chemistry, where precise knowledge of NaOH concentration is essential for titrations and other quantitative analyses.
-*Acid-base titrations
The reaction between KHP and NaOH forms a neutral solution, making it suitable for use as an indicator in acid-base titrations. The endpoint of the titration can be easily detected by the color change of an indicator added to the solution.
Food Industry
-*Leavening agent
KHP is used as a leavening agent in baking to produce carbon dioxide gas, which causes baked goods to rise. It is commonly employed in combination with sodium bicarbonate to achieve a consistent and controlled rise.
Medicine and Healthcare
-
-*Buffer solutions
KHP-NaOH mixtures can be used to create buffer solutions with a specific pH range. These buffers are essential for maintaining stable pH conditions in various biological and chemical processes, such as enzyme reactions and drug formulations.
-*Antacid
KHP can act as an antacid, neutralizing stomach acid to relieve discomfort caused by heartburn or indigestion.
Limitations
-
-*Temperature dependence
The KHP-NaOH reaction is temperature-dependent, meaning that the equilibrium constant and reaction rate change with temperature. This can affect the accuracy of analytical measurements and the efficiency of leavening in baking.
-*Reversibility
The reaction is reversible, meaning that the products (KNaC4H4O6 and H2O) can react to form the reactants (KHP and NaOH) under certain conditions. This reversibility can limit the effectiveness of the reaction in some applications.
Questions and Answers
What is the significance of the KHP-NaOH reaction?
The KHP-NaOH reaction plays a crucial role in analytical chemistry, particularly in acid-base titrations. It serves as a reliable method for determining the concentration of unknown acids by neutralizing them with a known concentration of NaOH solution.
How is the net ionic equation for the KHP-NaOH reaction derived?
The net ionic equation is obtained by removing the spectator ions from the balanced chemical equation. Spectator ions are ions that do not participate in the chemical reaction and remain unchanged throughout the process.
What are the practical applications of the KHP-NaOH reaction?
The KHP-NaOH reaction finds numerous practical applications, including standardizing solutions, calibrating pH meters, and analyzing the acidity of various substances. It is also used in environmental monitoring to determine the concentration of acids in water and soil samples.